The behavior of gases can be described by several laws based on experimental observations of their properties. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (Amontons's law). The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (Charles's law). The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant (Boyle's law).
Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules (Avogadro's law). Eventually, these individual laws were combined into a single equation—the ideal gas law—that relates gas quantities for gases and is quite accurate for low pressures and moderate temperatures. We will consider the key developments in individual relationships , then put them together in the ideal gas law. As with w/v solutions, we weigh out a specific amount of chemical when making a molar solution. Unlike w/v solutions, the amount to weigh depends on the molecular weight (m.w.) of the substance in grams per mole (g/mol). In order to calculate the desired mass of solute you will need to know the formula weight.
Formula weights are usually printed on the label and identified by the abbreviation f.w. Formula weight is the mass of material in grams that contains one mole of substance, and may include inert materials and/or the mass of water molecules in the case of hydrated compounds. For pure compounds the formula weight is the molecular weight of the substance and may be identified as such. In contrast to other gas components, water content in air, or humidity, to a higher degree depends on vaporization and condensation from or into water, which, in turn, mainly depends on temperature. Therefore, when applying more pressure to a gas saturated with water, all components will initially decrease in volume approximately according to the ideal gas law. However, some of the water will condense until returning to almost the same humidity as before, giving the resulting total volume deviating from what the ideal gas law predicted.
Conversely, decreasing temperature would also make some water condense, again making the final volume deviating from predicted by the ideal gas law. Perhaps the easiest way to describe a solution is in terms of weight-in-weight (w/w). The weight of the solute relative to the weight of the final solution is described as a percentage. For example, suppose you have a dye that is soluble in alcohol. Rather than write the instructions, "take 3 grams dye and mix with 97 grams absolute alcohol," you can describe the solutions simply as 3% dye in absolute alcohol. The formula applies to any volume of solution that might be required.
Three grams dye plus 97 grams alcohol will have final weight of 100 grams, so the dye winds up being 3% of the final weight. Note that the final weight is not necessarily equal to the final volume. Aqueous weight-in-weight solutions are the easiest to prepare. Since 1 milliliter of water weighs one gram, we can measure a volume instead of weighing the solvent.
A very common use of w/w formulas is with media for the culture of bacteria. Such media come in granular or powdered form, often contain agar, and often require heat in order to dissolve the components. Microbiological media, especially when they contain agar, are difficult to transfer from one vessel to another without leaving material behind.
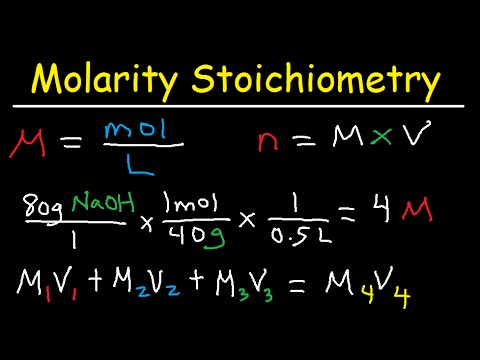
They coat the surfaces of glassware, making quite a mess. Using a w/w formula the media and water can be mixed, heated, then sterilized, all in a single container. Very little material is wasted and there is less of a mess. It is important to note that the molarity is defined as moles of solute per liter of solution, not moles of solute per liter of solvent. This is because when you add a substance, perhaps a salt, to some volume of water, the volume of the resulting solution will be different than the original volume in some unpredictable way.
To get around this problem chemists commonly make up their solutions in volumetric flasks. These are flasks that have a long neck with an etched line indicating the volume. The solute is added to the flask first and then water is added until the solution reaches the mark. The flasks have very good calibration so volumes are commonly known to at least four significant figures. Charles' law is a special case of the ideal gas law in which the pressure of a gas is constant.
Charles' law states that volume is proportional to the absolute temperature of a gas at constant pressure. Doubling the temperature of gas doubles its volume, so long as the pressure and quantity of the gas are unchanged. This relationship between temperature and pressure is observed for any sample of gas confined to a constant volume.
An example of experimental pressure-temperature data is shown for a sample of air under these conditions in Figure 9.11. The volume and temperature are linearly related for 1 mole of methane gas at a constant pressure of 1 atm. If the temperature is in kelvin, volume and temperature are directly proportional. Charles's law states that the volume of a given amount of gas is directly proportional to its temperature on the kelvin scale when the pressure is held constant.
Gases whose properties of P, V, and T are accurately described by the ideal gas law are said to exhibit ideal behavior or to approximate the traits of an ideal gas. An ideal gas is a hypothetical construct that may be used along with kinetic molecular theory to effectively explain the gas laws as will be described in a later module of this chapter. Although all the calculations presented in this module assume ideal behavior, this assumption is only reasonable for gases under conditions of relatively low pressure and high temperature. In the final module of this chapter, a modified gas law will be introduced that accounts for the non-ideal behavior observed for many gases at relatively high pressures and low temperatures.
As you mixed the two liquids, the ethanol and water molecules began to interact with each other, packing together in a different way than in either pure ethanol or pure water. This results in a new density of the solution and thus, a volume that does not equal the sum of the two original volumes. In other words, the volume of solution, the denominator of our volume percent equation, does not equal the volume of solute plus the volume of solvent.
In the case of water and ethanol, their mixture has less volume than the sum of the pure liquids. A disadvantage of describing formulas as w/v (%) is that the description says nothing about the actual concentration of molecules in solution. What if we want equal amounts of two chemicals to be mixed together, so that for each molecule of substance #1 there is a single molecule of substance #2? The same amount in grams will likely not contain the same number of molecules of each substance. For example, you may work with a chemical that can be in one of several forms of hydration. Calcium chloride can be purchased as a dry chemical in anhydrous form, so that what you weigh out is nearly all pure calcium chloride.
On the other hand you may have a stock of dry chemical that is hydrated with seven water molecules per molecule of calcium chloride. The same mass of this chemical will contain fewer molecules of calcium chloride. When we are interested in the actual concentration of molecules of a chemical in solution, it is better to have a universal measurement that works regardless of how the chemical is supplied. As long as the molecular weight is known, we can describe a solution in the form of moles per liter, or simply molar .
1 mole of an ideal gas is compressed isothermally from a volume of 500 cm3against a constant external pressure of 1.216 × 105 Pa. The pressure-volume work involved in the process is 36.5 J. Energy is transferred along with the genetic material and so obeys the first law of thermodynamics. Energy is transferred—not created or destroyed—in the process.
When work is done on a cell or heat transfers energy to a cell, the cell's internal energy increases. When a cell does work or loses heat, its internal energy decreases. In thermodynamics, the volume of a system is an important extensive parameter for describing its thermodynamic state.
The specific volume, an intensive property, is the system's volume per unit of mass. Volume is a function of state and is interdependent with other thermodynamic properties such as pressure and temperature. For example, volume is related to the pressure and temperature of an ideal gas by the ideal gas law.
When we describe a concentration as a percentage without specifying the type of formula, we imply that the solution is to be made using the weight-in-volume (w/v) method. As with w/w, weight-in-volume is a simple type of formula for describing the preparation of a solution of solid material in a liquid solvent. Boyle's law, together with Charles's law and Gay-Lussac's law, are among the fundamental laws which describe the vast majority of thermodynamic processes. We have gathered them all in our thermodynamic processes calculator, where you can choose whichever process you like, and evaluate the outcomes for a real gases.
Calculate the final volume of one mole of an ideal gas initially at 0oC and 1 atm pressure. If it absorbs 2000 cal of heat during reversible isothermal expansion. Human metabolism is the conversion of food into energy given off by heat, work done by the body's cells, and stored fat. Metabolism is an interesting example of the first law of thermodynamics in action. Eating increases the internal energy of the body by adding chemical potential energy; this is an unromantic view of a good burrito. 3 moles of an ideal gas are expanded isothermally from volume of 300 cm3to the volume 2.5 L against a constant external pressure of 1.9 atm at 300 K.
Consider an ideal gas having definite mass be enclosed in a cylinder fitted with weightless, frictionless, tightly fitted, movable piston. Let 'A' be the area of the cross-section of the cylinder. Let the gas expand from volume V1 to V2 against constant external pressure Pext which is exerted on the piston. Due to expansion, the piston moves upward by a distance 'd'. In this process, the system loses energy to the surroundings. Most chemicals tend to absorb water unless they are kept desiccated, that is to some extent they are hydroscopic.
This problem should not be confused with the state of hydration of a substances, which refers to the direct association of water molecules with molecules of the substance through hydorgen bonding. Magnesium chloride is commonly used in biological buffers, and is notoriously hydroscopic. It is usually not practical to worry about water content, since it is so difficult to control.
If precision is critical, then chemicals should be maintained under desiccating conditions or used immediately before they can absorb a significant amount of water. Suppose that someone has already worked out the details, so all that you have to do is read a formula and make a solution. We can usually assume that a solution is to be aqueous unless stated otherwise.
What about the concentration of the substance to be added? Common ways of describing the concentrations of solutions are weight-in-weight, weight-in-volume, volume-in-volume, and molarity. Less commonly used descriptions include normality and molality. A quantity of solute is measured out, mixed with solvent, and the volume is brought to some final quantity after the solute is completely dissolved.
That is, solutions are typically prepared volumetrically. Because solutes add volume to a quantity of solvent, this method of preparation of solutions is necessary to ensure that an exact desired concentration is obtained. If we partially fill an airtight syringe with air, the syringe contains a specific amount of air at constant temperature, say 25 °C. This example of the effect of volume on the pressure of a given amount of a confined gas is true in general.
Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. In fact, if the volume increases by a certain factor, the pressure decreases by the same factor, and vice versa. Volume-pressure data for an air sample at room temperature are graphed in Figure 9.13. The line stops at 111 K because methane liquefies at this temperature; when extrapolated, it intersects the graph's origin, representing a temperature of absolute zero. The ideal gas equation contains five terms, the gas constant R and the variable properties P, V, n, and T. Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example exercises.
Volume-pressure data for an air sample at room temperature are graphed in Figure 5. Pour the tinted water into the 500-mL volumetric flask, using a funnel if needed. Pour the tinted ethyl alcohol into the 500-mL volumetric flask, using a funnel if needed. Note the level of the liquid in the 500-mL volumetric flask. (It should be very close to the 500-mL volume line.) Place a stopper or cap on the 500-mL volumetric flask and invert the flask several times. Remove the stopper or cap to prevent a vacuum from occurring inside flask.
Replace the cover and continue mixing the solution by inverting the flask until a green color is consistent throughout the solution. Allow the solution to settle, then observe the volume of liquid. (It should be far below the 500-mL volume line.) Mass the 500-mL volumetric flask containing the water-alcohol solution. Determine how much volume was lost in the creation of this solution. Using a disposable pipet, add water from a graduated cylinder to the volumetric flask containing the water-alcohol solution. Subtract this volume from 500 mL to obtain the final volume.
Ions with large hydrophobic surfaces such as tertaalkylammonium ions in effect increase hydrogen bonding in the adjacent water structure, which entails a further contribution, V∞caged. If this effect prevails, the ion is a structure-making ion, in contrast to the structure-breaking ions with a large V∞disorder term. Similarly, the appropriate value of heat capacity to use in a given process depends on whether the process produces a change in volume. The heat capacity is a function of the amount of heat added to a system.
In the case of a constant-volume process, all the heat affects the internal energy of the system (i.e., there is no pV-work, and all the heat affects the temperature). 1 mole of an ideal gas is compressed isothermally from volume of 20 L to 8 L against constant external pressure, when pressure volume work obtained is 44.9 L atm. This is an expression for pressure volume work done in terms of pressure and volume in the isothermal expansion of an ideal gas against constant external pressure. The volume units must be the same for both volumes in this equation. In general, M1 usually refers to as the initial molarity of the solution.
M2 refers to the final concentration of the solution and V2 is the final total volume of the solution. Imagine filling a rigid container attached to a pressure gauge with gas and then sealing the container so that no gas may escape. If the container is cooled, the gas inside likewise gets colder and its pressure is observed to decrease.